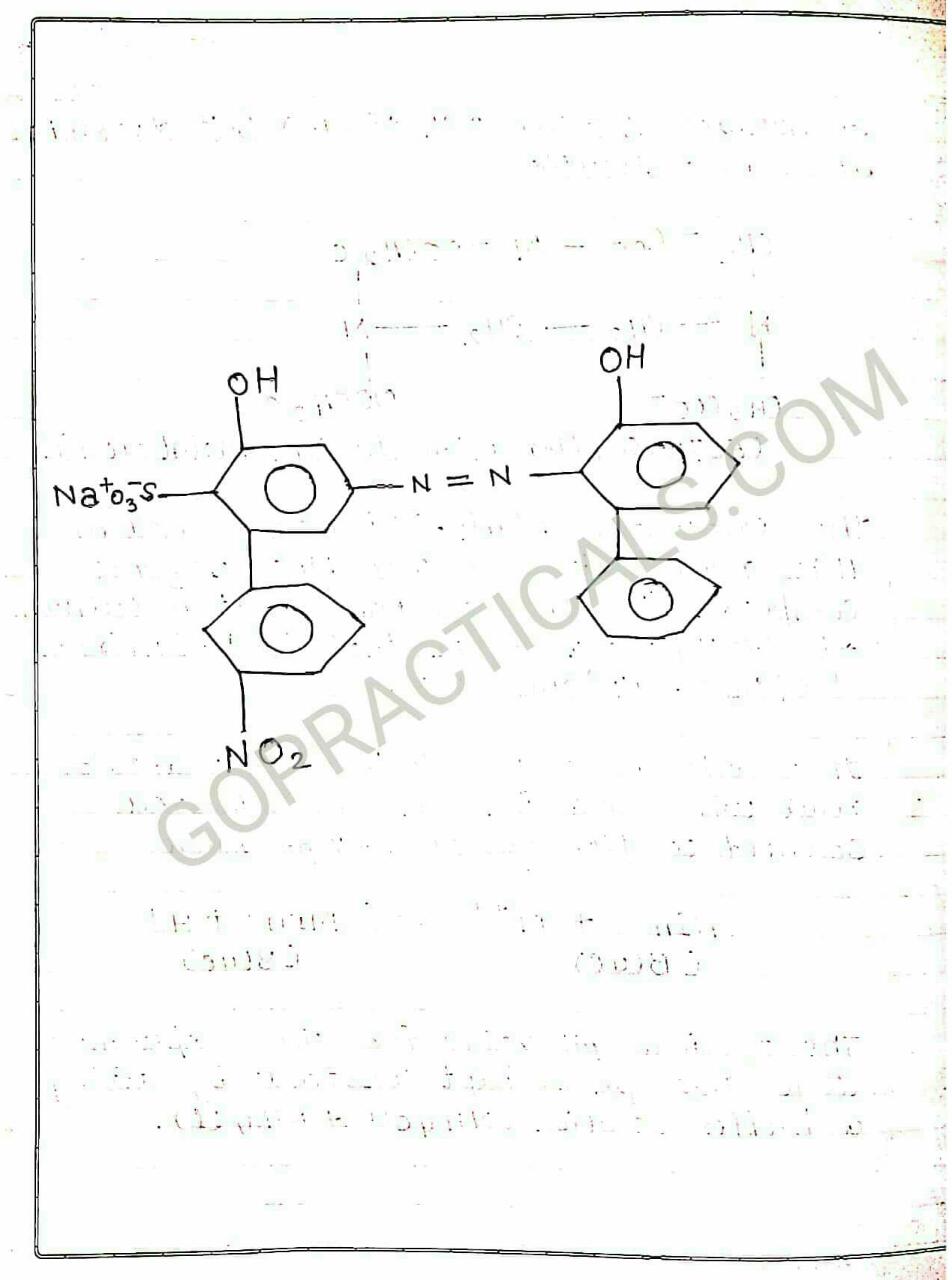
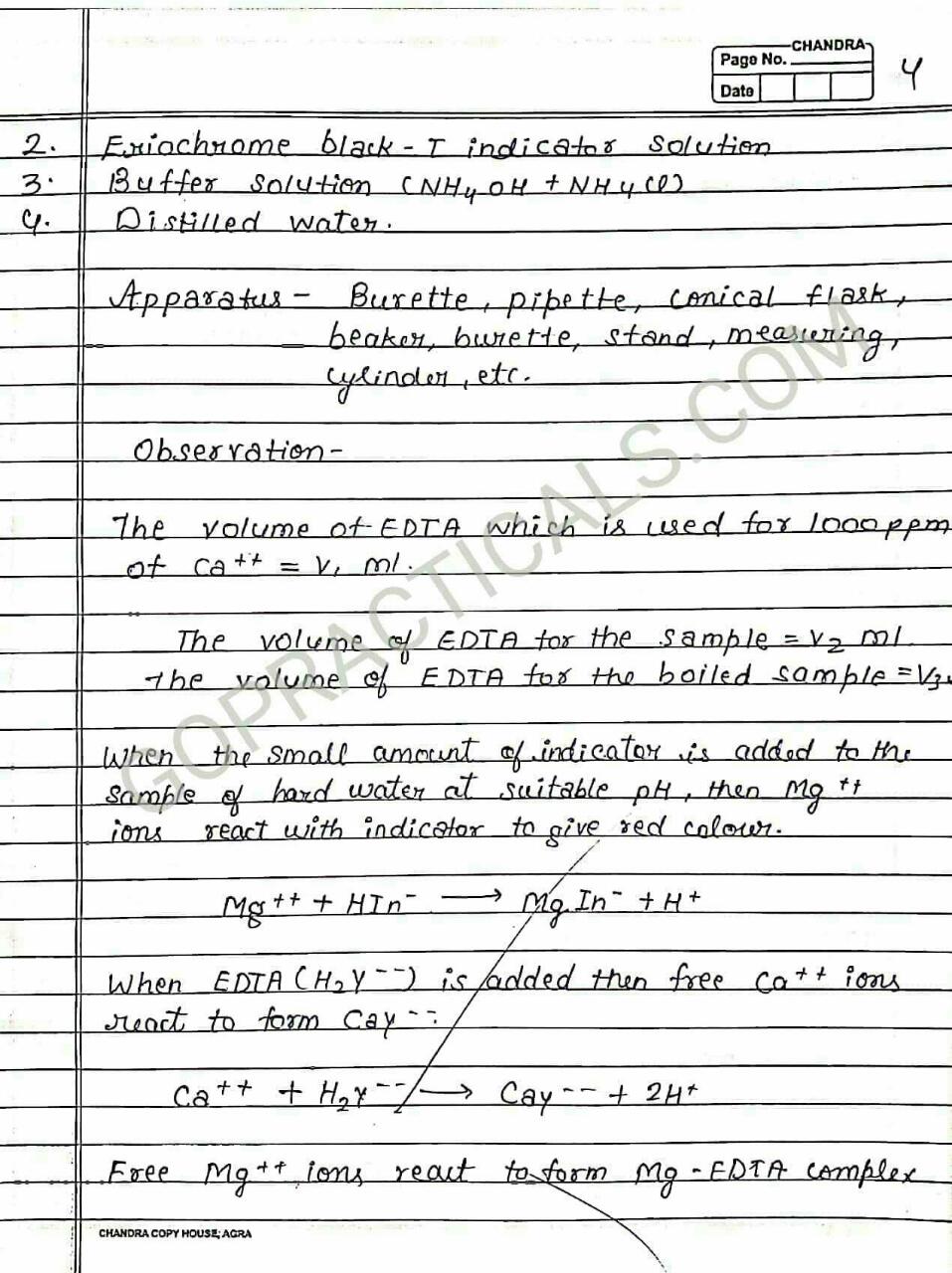
Eriochrome Black T is a complexometric indicator that is used in complexometric titrations, which is mainly used to determine total hardness of Water. In the below photo you can see that in its deprotonated form, Eriochrome Black T is blue. It turns red when it forms a complex with calcium, magnesium, or other metal ions.

Practical to Determine total hardness of water sample in terms of Caco3 by EDTA Titration method using Eriochrome black T indicator





Watch this Video to know more about Eriochrome black T indicator and how to calculate hardness of Water
Also Check Out – Basic Chemical Practicals
Thanks for visiting us…
To Determine total hardness of Water sample in terms of Caco3 by EDTA Titration method using Eriochrome black T indicator – Chemical Practical